contamination and cross contamination, prevention of cross contamination
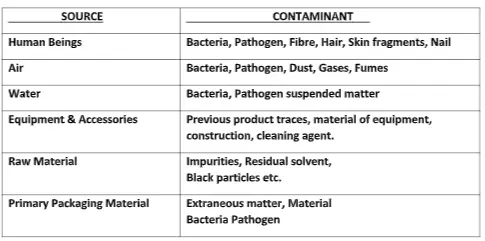
Contaminants: Contaminants are any impurity, Product, or substance other than product manufacturing. Foreign products, Particulate matter, Microorganisms, Endotoxin (degrade microorganisms), Cross-contamination is a case of contamination.
The Peoples generated contaminant:
- Our outer skin is completely shed every 24 hours.
- Particles of 0.3micron and greater are liberated at a rate varying between 100000 to 10 million per minute.
- A person walking will liberate thousands of bacteria per minute, and a single sneeze can be produced up to 1 million bacterias.
- The manufacturing process itself can generate, for example, paint off from equipment, dust from belt drives, etc.
Contamination: “The undesired introduction of the chemicals or a microbial nature, or of foreign matter into or on to a starting material or intermediate, during production, sampling, packaging or repackaging, storage, and transport.”
Types of contamination:
- Chemical contamination (leftover residue of the previous process)
- Microbiological contamination ( microbiological flora)
- Particulate contamination ( particles like dust, fibers, oil, or grease from the pieces of equipment. others like haires, skin fragments.)
Cross-contamination is the contamination of the starting, intermediate products, or finished products with other starting materials or products during production.
From where does cross-contamination generate?
- It may be due to poor design and operated air handling systems and dust extraction systems.
- Inadequate procedure for personnel and equipment
- Not proper cleaning of equipment.
How to minimize cross-contamination?
- Use of closed production systems
- By applying a validated cleaning procedure
- By maintaining a level of product manufacture
- Adequate premises
- By installing of correct air pressure cascade
How to control dust in pharmaceuticals: Dust control is very necessary to control contamination and cross-contamination in pharmaceuticals. Whenever possible, the dust or vapor contamination should be removed at the source.
Dust extraction duct should be designed with sufficient transfer velocity to ensure dust is carried away. Airflow direction should be carefully chosen that the operator does not contaminate the Product. Use of well-validated HEPA filters in series to provide additional protection. A sophisticated computer-based data monitoring system may be installed.
Conclusion:
The system or equipment shall be validated to prevent the Product from contamination and cross-contamination. Cleaning validation of equipment and area with a well-defined procedure shall be maintained. Using the cleaning validation approach, we can easily identify any discrepancy or loops in a system.
Pharmaceuticals are required to the validated cleaning procedure, AHU system, air cascade, and personnel movements to ensure the production of good quality products throughout its life cycle. Failure to prevent contamination and cross-contamination can lead to a loss in customer faith or satisfaction from the investors, loss of market values, loss in money, and direct consequences from regulatory bodies.
Comments
Post a Comment